Fire is a fascinating element that has intrigued humans for centuries. Have you ever wondered if fire really needs oxygen to burn? In this article, I’ll delve into the science behind fire and its relationship with oxygen. Understanding this fundamental concept can shed light on how fire behaves and how we can control it.
As I explore the question of whether fire needs oxygen, we’ll uncover the crucial role that oxygen plays in the combustion process. From the ignition to the flame’s sustenance, oxygen is a key player in fueling the fire. By grasping this connection, we can gain a deeper appreciation for the chemistry of fire and the importance of oxygen in this fiery phenomenon.
Key Takeaways
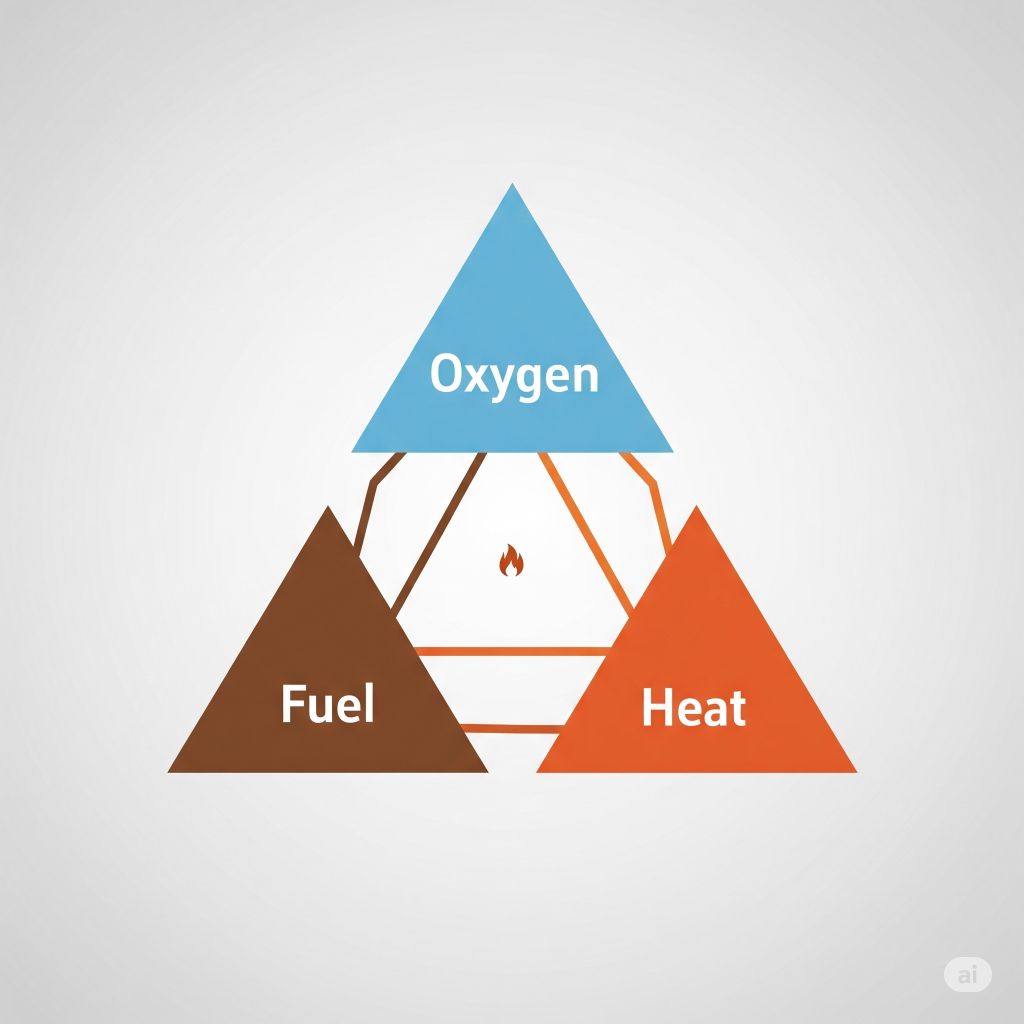
- Fire requires three essential components to burn: fuel, heat, and oxygen, known as the “fire triangle.”
- Oxygen is a crucial element in the combustion process, acting as the oxidizing agent that sustains the flame.
- Proper ventilation is essential to ensure an adequate oxygen supply for maintaining a healthy and robust flame.
- Oxygen influences the rate of burning in a fire and plays a key role in fire ignition and sustainability.
- Understanding the chemistry of fire and the role of oxygen is fundamental for fire safety measures and efficient fire management.
- Oxygen not only supports combustion but also impacts the intensity and consistency of a fire, emphasizing its significance in fire behavior and control.
The Science Behind Fire
When we talk about fire, we are diving into the realm of combustion – a fascinating chemical process that releases heat and light. For a fire to ignite and sustain itself, it requires three essential components: fuel, heat, and oxygen. These elements come together in what is known as the “fire triangle.”
Fuel is the material that undergoes combustion, releasing energy in the form of heat and light. Heat is the energy needed to raise the fuel to its ignition temperature. Oxygen, on the other hand, is crucial for the combustion process, acting as the oxidizing agent that sustains the flame.
In a nutshell, the combustion process involves the fuel combining with oxygen in the presence of heat. This chemical reaction produces heat, light, and various byproducts like smoke and ash. Without one of these components, the fire cannot exist or will extinguish.
Understanding the science behind fire not only sheds light on its mesmerizing nature but also underscores the importance of safety measures. By comprehending the role of oxygen in combustion, we gain a deeper appreciation for the controlled use of fire in various aspects of our lives, from cooking and heating to industrial processes and transportation.
The Role of Oxygen in Combustion
When it comes to the question, “Does fire need oxygen?” the answer lies in understanding the role of oxygen in the combustion process. Oxygen is a critical component in the fire triangle, being essential for sustaining a fire.
During combustion, the fuel combines with oxygen in the presence of heat to produce heat, light, and byproducts. This chemical reaction is exothermic, meaning it releases heat as it progresses. Without oxygen, this reaction cannot occur, and thus, fire cannot be sustained.
In simple terms, oxygen acts as the oxidizer in the combustion process. It helps break down the fuel molecules and releases energy in the form of heat and light. The higher the concentration of oxygen, the more efficiently the combustion process can take place.
In everyday scenarios, ensuring proper ventilation is crucial when dealing with fire. Adequate oxygen supply not only sustains the combustion process but also prevents the accumulation of harmful byproducts that can result from incomplete combustion.
Moreover, industrial processes often rely on controlled oxygen flow to regulate combustion efficiency and minimize emissions. Understanding the role of oxygen in combustion is not only fascinating but also vital for maintaining safety and optimizing the use of fire in diverse applications.
Ignition: Oxygen’s Key Role
When it comes to the question, “Does fire need oxygen?” the answer is a resounding yes. Oxygen fuels the flames by combining with the fuel source in a process known as oxidation. This reaction releases heat and light energy, sustaining the fire’s combustion. Without oxygen, the fire would lack a vital ingredient for its existence.
In the fire triangle, oxygen plays a crucial role as the oxidizer. Most fires require oxygen levels of at least 16% to sustain combustion. Without sufficient oxygen, the fire’s intensity diminishes or may even be extinguished. Proper ventilation is essential in ensuring that fires receive an adequate oxygen supply to burn brightly and consistently.
Not only does oxygen support the combustion process, but it also influences the rate of burning. The amount of oxygen present affects the speed at which the fire spreads. By controlling the oxygen supply, individuals can manage the fire’s intensity and prevent it from growing uncontrollably.
oxygen stands at the core of fire ignition. Its presence is indispensable for the sustained burning of fuel sources and the generation of heat and light. Understanding the pivotal role of oxygen in fire’s existence is fundamental to fire safety practices and efficient fire management.
Sustaining the Flame: Oxygen’s Influence
When discussing whether fire needs oxygen, it’s crucial to understand oxygen’s influence in sustaining a flame. Oxygen plays a vital role in the combustion process by combining with the fuel source to produce heat and light energy. This chemical reaction is essential for the continued existence of a fire.
Proper ventilation is key to ensuring that fires receive an ample oxygen supply for consistent burning. Without adequate ventilation, a fire may smolder or struggle to sustain itself due to a lack of oxygen. This highlights the importance of oxygen in maintaining a healthy and robust flame.
Moreover, the presence of oxygen not only supports combustion but also significantly impacts the burning rate of a fire. The rate at which fuel burns and heat and light are generated is directly influenced by the availability of oxygen. In essence, oxygen acts as a catalyst, accelerating the burning process and promoting a more efficient release of energy.
Understanding and recognizing oxygen’s pivotal role in fire ignition is crucial for implementing effective fire safety measures and efficient fire management practices. By acknowledging the significance of oxygen in sustaining a flame, we can better comprehend the dynamics of fire behavior and enhance our overall approach to fire prevention and control.
Conclusion
Oxygen plays a vital role in sustaining fires by combining with the fuel source to produce heat and light energy. Adequate ventilation is crucial for ensuring consistent burning and preventing smoldering. Understanding oxygen’s impact on fire burning rates is key for effective fire safety measures and management practices. By recognizing oxygen’s pivotal role in fire ignition, we can enhance our understanding of fire behavior dynamics and improve fire prevention and control strategies.
Frequently Asked Questions
Why is oxygen important in sustaining a flame?
Oxygen plays a crucial role in sustaining a flame by combining with the fuel source to produce heat and light energy, which are essential for combustion.
Why is proper ventilation essential for fires?
Proper ventilation is vital to ensure fires receive sufficient oxygen for consistent burning. Inadequate ventilation can lead to smoldering or difficulty in sustaining the flame.
How does oxygen impact the burning rate of a fire?
Oxygen acts as a catalyst to accelerate the burning process and enhance energy release efficiency, significantly impacting the burning rate of a fire.
